Have a question? 06 70 73 89 02
🔞 Not for sale to under 18s
🔥 30% off EVERYTHING on the site with the code: LOVE!!! 🔥 ( except accessories and gummies)
Have a question? 06 70 73 89 02
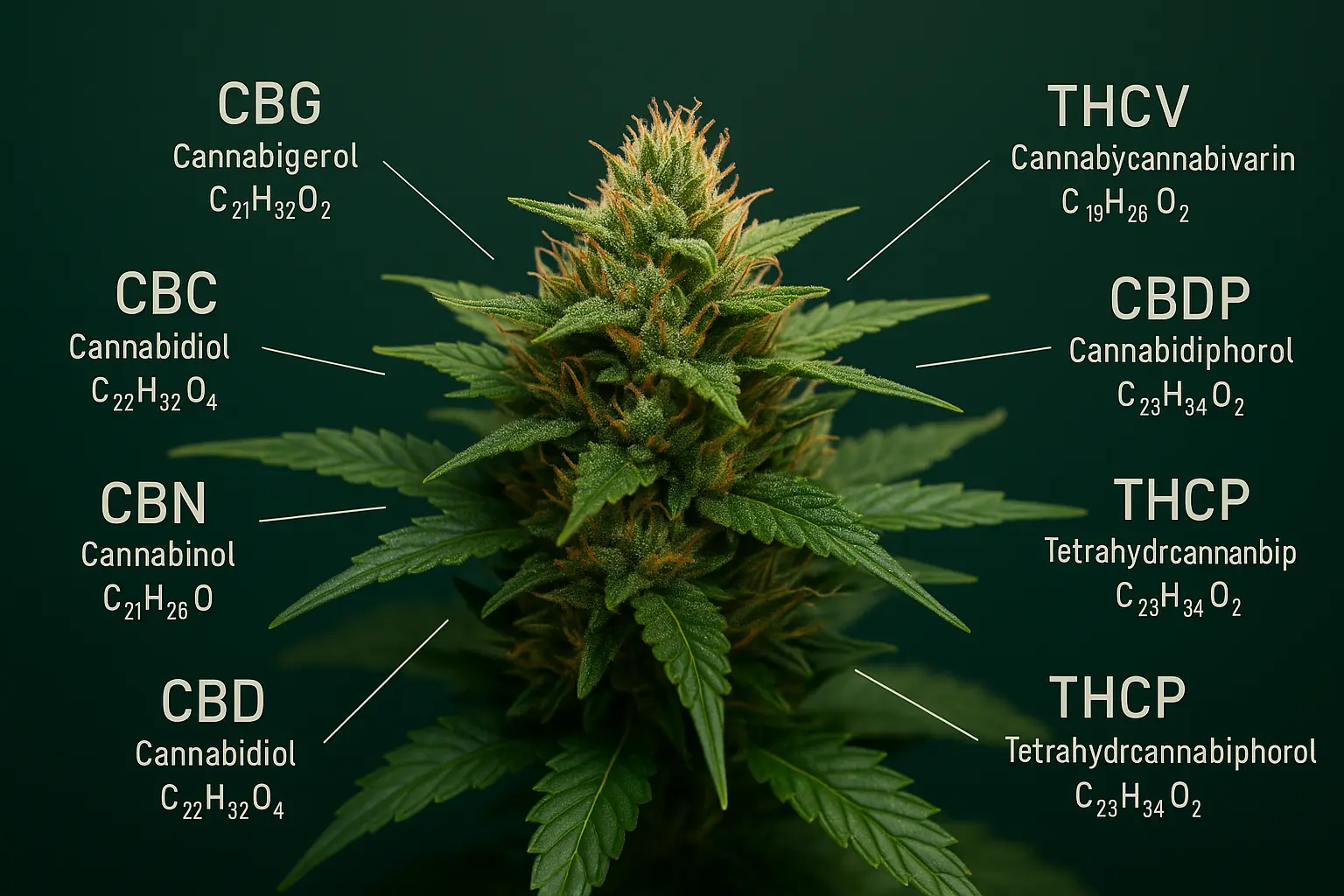
CBD, CBG, CBC, CBN... Having trouble finding your way around all these acronyms? Well, there are over 100 natural cannabinoids synthesized by cannabis.
But it doesn't have to be that way either: to help you find your way around, we've put together a list of the best-known natural cannabinoids, with their full names, acronyms, effects and a few extra bits of information to help you shine in society!
Natural cannabinoids, also known as phytocannabinoids, are molecules produced by plants in the Cannabis genus. This genus includes the sativa, indica and ruderalis varieties, all of which belong to the Cannabaceae botanical family.
Natural" cannabinoids are those produced directly by the plant through its biological processes, without human intervention. These are the well-known substances found in cannabis and hemp flowers and resins: CBD, THC, CBG, CBC and others.
The terms major cannabinoids and minor cannabinoids are totally artificial groups, created by the scientific literature for classification purposes.
These are cannabidiol (CBD), tetrahydrocannabinol (THC), cannabigerol (CBG) and cannabinol (CBN).
By exclusion, all other cannabinoids are said to be "minor" or "secondary".
Each cannabinoid has its own particularities, but many share a similar chemical structure: they all belong to the large phytocannabinoid family, derived from the same biosynthetic pathway.
Most phytocannabinoids are synthesized during the growth and drying of cannabis flowers.
It's a complex biological process. To put it simply, the plant first produces a key molecule: CBGA (cannabigerolic acid), often referred to as the "mother molecule".
Depending on the plant's genetics, larger or smaller portions of CBGA are converted into other cannabinoid acids by specific enzymes.
The result is different levels of THCA, CBDA, CBCA, etc.
These acidic forms are then transformed into the "classic" cannabinoids we know as THC, CBD, CBG, etc., during flower drying.
Now that we've seen what a cannabinoid is and how it's created, let's take a closer look at each cannabinoid in detail.
We start with the best-known cannabinoid: THC. Scientifically, it's called Delta-9 THC, to distinguish it from other forms of THC present but much less concentrated, such as Delta-8thc and Delta-10 THC.
THC is known for its action on the endocannabinoid system, in particular on CB1 receptors, which gives it its well-known psychoactive effects:
But THC is also known for its addictive effects, as well as its undesirable side effects (anxiety, paranoia...) which justify its almost worldwide classification as a narcotic.
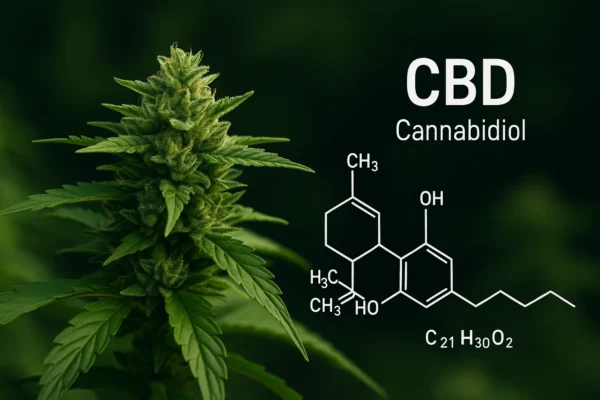
Second on our list is, of course, CBD. This cannabinoid is the second most concentrated in cannabis, particularly in indica flowers.
Unlike THC, it does not interact directly with the endocannabinoid system, which partly explains the difference in effects. Cannabidiol doesn't produce a high, but it can produce a wide range of effects. Among these, the most frequently cited are :
Cannabidiol has a modulating effect on biological equilibrium, and as such is recognized by most European countries as a safe cannabinoid.
Although it was the first cannabinoid to be created in acid form, CBG is actually quite rare in CBD flowers. In most cases, it represents less than 1% of the cannabinoids present.
Like CBD, CBG acts only weakly on the endocannabinoid system, giving it modulatory effects on the system. Cannabigerol is generally described as more stimulating than CBD, and opinions speak for themselves:
It may also help promote well-being and emotional balance.
CBN is a particularly interesting cannabinoid, since it is not produced directly by the plant. It arises naturally from the breakdown of THC, as the latter ages or oxidizes under the effect of heat, light or oxygen.
Schematically, tetrahydrocannabinol (THC) and cannabinol (CBN ) share a very similar structure: CBN can be seen as a more oxidized, "older" form of THC. Over time, THC loses certain hydrogen atoms and its cycle becomes rigid, leading to the natural formation of CBN.
This oxidation process explains why more CBN is found in flowers or resins that have been stored for a long time or exposed to heat.
Although derived from THC, the effects of Cannabinol are totally different. With a very low high, it doesn't induce euphoria. It is known for its sedative effects, which would make it a good candidate for helping to find sleep.
CBC is probably one of the cannabinoids that attracts the least attention, for one simple reason: it produces few perceptible effects on its own.
With virtually no binding to the CB1 receptors of the endocannabinoid system, CBC is often described as giving a very subtle sensation of calm, even more discreet than CBD.
In fact, some studies suggest that CBC may play a role in the entourage effect : its presence would modify the way other cannabinoids are experienced, in particular by softening or balancing some of THC's effects.
It's a "background" cannabinoid, unspectacular on its own, but contributes to the overall effect of a flower or resin.
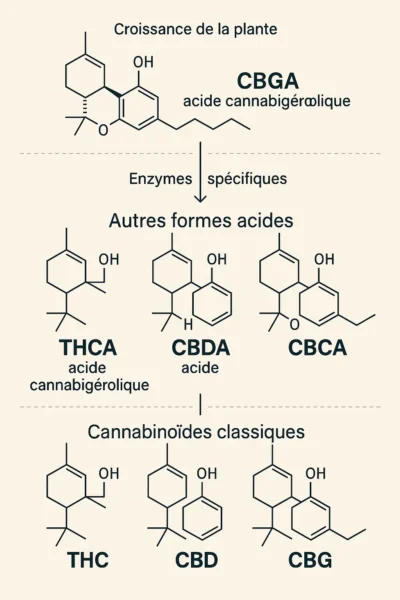
As we saw in the section on the formation of natural cannabinoids, before becoming the molecules we know today, cannabinoids first existed in acid form: CBGA, THCA, CBDA, CBCA, among others.
Essentially present in fresh flowers, these acidic forms have long been considered "inactive", as they produce little or no perceptible effects.
Yet recent research has shown that these acidic molecules have their own biological effects, sometimes even more targeted than their decarboxylated versions. Some have marked anti-inflammatory, antioxidant or anti-nausea properties, without any psychoactive effect or change in perception.
They therefore represent a very interesting avenue for medical research. But a great deal of research is still needed to fully understand their potential and their specific mechanisms.
That's right! Although this group of cannabinoids was mostly known thanks to chemically-produced THCV, which became popular before it was banned in 2023, cannabinoids of the varine group exist naturally. That's why natural THCV has been requalified and is still legal at concentrations below 0.3%.
Cannabinoids whose names end in -varine (such as THCV, CBDV, CBCV, CBGV, to name but a few) are quite similar to their "classic" versions.
Most of them differ molecularly only in that they have a shorter side chain: a simple structural change, but one that influences their biological behavior, as well as their potential and felt effects.
They are generally thought to have a brighter, less long-lasting effect than their classic equivalents.
Recently discovered in 2019, the so-called phorol or biphorol forms represent another rare family of natural cannabinoids. They can be seen as the "inverse twins" of the -varine forms.
Where -varines have a shorter side chain (C3), biphorols have a longer one (C7). This simple elongation modifies their biological behavior: it strengthens the bond with endocannabinoid receptors, which can influence the nature and intensity of their effects.
These molecules do exist naturally in certain varieties of hemp, but in minute quantities, often of the order of 0.01% or less. Their recent discovery has led to a better understanding of the fundamental role of side-chain length in cannabinoid activity.